Background: Topically active corticosteroids have been shown to effectively treat inflammatory bowel disease with a systemic steroid-sparing effect. However, previous studies have not demonstrated their efficacy in preventing graft-versus-host disease (GVHD) when combined with the standard GVHD prophylaxis regimen consisting of calcineurin inhibitors and methotrexate. We hypothesized that the addition of orally administered ileal-controlled-release budesonide to the post-transplant cyclophosphamide (PTCy)-based regimen could effectively prevent severe acute gastrointestinal GVHD. In this study, we present outcomes of the first 43 consecutively treated patients (pts) with the PTCy-tacrolimus (T), mycophenolate mofetil (M) and budesonide (B) (PTCy-TMB) as a GVHD prophylaxis regimen after allogeneic stem cell transplantation (alloSCT).
Methods: Pts with hematologic malignancies received their first alloSCT at our institution between 05/2020-04/2023. Thirty one pts received conditioning with fludarabine (160 mg/m 2) + melphalan (100-140 mg/m 2) + TBI (2-4Gy) or thiotepa 5 mg/kg. Twelve pts received fludarabine (150mg/m 2) + cyclophosphamide (29mg/kg) and TBI 4Gy ± ATG (4.5 mg/kg). GVHD prophylaxis consisted of Cy (50 mg/kg on D+3 and +4), T (trough goal 6-10 ng/mL), M (15mg/kg TID) and budesonide (3 mg TID) starting on D+5 and continued until D+90 followed by a weekly taper if no acute GVHD (aGVHD) occurred. T was continued for at least 6 months followed by a taper, unless otherwise indicated.
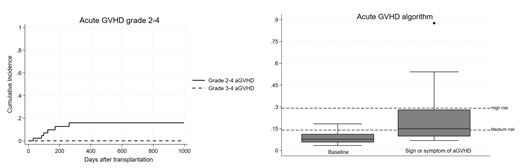
Results: The median age was 50 years (range 22-73), 39 pts had hematologic malignancies including AML (N=14), ALL (N=11) and NHL (N=8). Seven pts (16%) classified as high/very-high DRI. The median of HCT-CI was 4 (range 0-7). Donors were haploidentical (N=32), MSD (N=8), MUD (N=1), MMSD (1) and MMUD (N=1). PBSCs were used in 28 pts (65%). Thirty pts (70%) received RIC regimens. Median follow-up was 407 days (range 91-996 days). At day 100, the cumulative incidence (CI) of grade 2 aGVHD was 7.0% (95%CI 1.8-17.2), while none developed grade 3-4 aGVHD (Figure 1). One patient had severe chronic GVHD (bronchiolitis obliterans) with CI at 2 years of 3.2% (95%CI 0-14.0).
The CI of any systemic steroid use at D+100 and D+180 was 30.2% (95%CI 17.4-44.1) and 44.8% (95%CI 28.9-59.5), respectively. Among the pts who received treatment for aGVHD, the median cumulative systemic steroid exposure (in prednisone equivalents) was 1,318 mg (IQR 623-3,775) at D+100 and 1,325 mg (IQR 525-1,640) at D+180, with a median treatment duration of 40 days (IQR 25-110).
At 1 year, the OS, PFS, GRFS, relapse and NRM were 79.6% (95%CI 61.4-89.9), 69.8% (95%CI 51.6-82.3), 66.6% (95%CI 48.4-79.8), 15.3% (95%CI 6.2-28.2) and 14.9% (95%CI 5.3-29.1), respectively. The 1-year CI of newly diagnosed/worsening hyperglycemia and Cushing syndrome was 18.2% (95%CI 7.9-31.9) and 5.0% (95%CI 0.9-14.2), respectively. 3 pts (7%) required insulin treatment.
The median absolute lymphocyte count (ALC) at day 30, 90 and 180 was 200/mcL, 300/mcL and 500/mcL, respectively, with no differences in the median ALC at D+180 among pts with and without GVHD. Median cell numbers of CD3 +, CD3 +/CD4 +, CD3 +/CD8 +, CD19 + and CD56 + at 1, 3, 6 months post-transplant were D+30 - 63/mcL, 18/mcL, 34/mcL, 1/mcL, 87/mcL, D+90 - 111/mcL, 64/mcL, 34/mcL, 9/mcL, 216/mcL and D+180 - 269/mcL , 78/mcL , 126/mcL , 7/mcL, 189/mcL, respectively.
After 10/2022 pts were tested routinely for Reg3α and ST2 (baseline and retested if developed any signs/symptoms of aGVHD). Among 18 tested pts at baseline, the median number (range) of Reg3α and ST2 at baseline was 28 ng/mL (16-90) and 23,308 pg/mL (4,678-56,226), respectively. Three low-risk pts developed grade 1 aGVHD (N=2) and grade 2 aGVHD (N=1). Fourteen pts with GI sign/symptoms after transplant showed median number (range) of Reg3α and ST2 of 78 ng/mL (16-837) and 34,904 pg/mL (13,329-160,000), respectively. Only one pt with high-risk aGVHD algorithm developed grade 2 aGVHD (Figure 1).
Conclusions: The addition of budesonide to PTCy-based GVHD prophylaxis regimen (PTCy-TMB) is feasible and appears to be effective in preventing severe GI aGVHD, without compromising transplant outcomes and immunologic reconstitution.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal